|
Fixing the broken Periodic Table for the world of
Chemistry.
Jim & Rhoda Morris
( A physicist & Chemist team)
What was the world of chemistry and the periodic
table like when Henry Gwyn Jeffreys Moseley,
a physics graduate student was looking around for a
project to do for his advanced degree in physics?
Well The world of chemistry was a poorly
organized muck of negatively and positively charged maybe particles.
At the same time while your searching with us look and
find if you can find some glimpse scatter through out this note,
high lighting how science and scientists work together to the benefit of all!. If the message comes
through can you
guess Why the proven productive rate of science is so much greater than other
professions. Why in the end and in spite of all of the obstacles, that some
would throw in science's path, it over comes them and keeps moving on
at a faster rate. Can we use science's secrets in other professions?
A National Science Foundation ...NSF ... funded video project titled the
Mystery of Matter.
The expressions and or errors in this note are solely those of the
authors and not those of the sponsors.
WHAT WERE
SOME OF THE HOTTEST QUESTIONS IN PHYSICS AND CHEMISTRY THAT NEEDED TO BE
SOLVED?
WHAT WAS AN ATOM MADE OF?
WHAT DID AN ATOM LOOK LIKE?
WHAT WERE THE CORRECT
ADDRESSES FOR ALL THE ATOM'S ... in the period table?
...
WAS THERE PROOF OF MISSING ATOMS?
WHICH OF THE NEW THEORIES ABOUT ATOMS WAS THE CORRECT ONE?
WERE THERE NEW INSTRUMENTS TO MEASURE AND ANSWER THESE QUESTIONS?
WHO WAS WORKING OR AVAILABLE TO WORK ON
THESE PROBLEMS?
In England some of the very active scientists
were working on the
questions above. They all came away with awards. They all
worked well together competitively and individually to get it right. All
scientist have to do this or they will simply get passed by those who do.
Here is a small sample of the motley crew that Henry had to deal with. They were all as
sharp as a tack and go getters.
|
Professor Lord Rutherford of Nelson author of a number of models of atoms
encouraged Moseley experimental work on the atom |

William L. Bragg X-ray crystallographer, co discoverer (with his Professor
father 1912) of the
Bragg
law of X-ray diffraction which Moseley depended on and used in his experiment. |
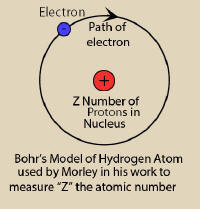
Meet mister Planetary Model of a hydrogen atom suggested by Bohr.
The single orbit shown above is just one of an infinite number
circular paths single electron can take. available. The one
proton can be lots of proton s |
 Niels Henrik
David Bohr an almost student corresponding and encouraged
Moseley about his work on the .atom which would experimentally
confirm his theoretical work.
|
|
AND |
THERE |
WERE |
T |
The authors, discovered during this project, a
pleasant and surprising technical connection between us (Jim and Rhoda)
and the scientists above. For several years we studying theoretically and
experimentally the broadening of spectral lines such as
hydrogen and heavier atoms that are under high pressures similar to stellar
atmospheres and a-bombs blasts. Here is a working example of the step by step
progress all scientist share in. There are no one man stands the giant of all
giants. No matter what one has been told and believes iit beyond all
reason.
Theoretician can and do build
great theoretical worlds which are internally consistent within themselves but
until they are experimentally verified several ways they are pure conjecture and
debate and have fun but don't take them even a little bit true.
Make sure that the picture includes that we made the unit
spectroscopic the same curious incidence showing how science works
that the authors had also worked a few years ago on the hydrogen spectra,
in particular the Beta line of Hydrogen. We were also checking theory, JUST like
Moseley, in our case we were testing for the spectral line shape and
line width of the Beta line in the visible spectra which was caused
by the interaction of the free electrons surrounding and disturbing hydrogen
atoms while they are radiating. Our worked was supported by the NASA/ Air Force
funded contract. Just like Moseley we also used a grating In our spectrograph.
But very important Moseley used a very special grating, one made by nature. it
was in crystalline form. It had just had been discovered and worked on by
in neighboring facilities by the Bragg's.
Below is one of many photos we took of Moseley's
equipment while visiting the Oxford Museum in Oxford England. Our
purpose was to study the original equipment Moseley used in
his ground breaking work on the atom. From experience we felt the
compelling need to get our information, not just from the professional
historians, but also first hand from an experienced
experimental physicist and a chemist's point of view ...
We did this to accurately replicate Moseley equipment ...and his
experimental technique, and to share this for the NSF project.
Hint, hint to be noted we asked others including the then National Bureau
of Standards to repeat our experiment, as is done, in all good science. They and
others did and concurred that our work was correct.
Our experiment was part of a much large body of work. It was titled (
Experimental Test of H Beta Stark Broadening Theory at High Electron Densities
J. C. Morris and R. U. Krey, Aug 19,1968 Physical Review Letters Vol. 21 Number
15 Physical Review Letters.
Back to the story and very important photo
showing something very important, more important than who did it.
For the photo below is one of a number of the actual Potassium
Ferrocyanide crystals that Moseley used in his work. This single item was the
most important of all of his equipment. It created the spectra not from a
prism but a very special grating( made out of a relative common crystal) for
analyzing the x-rays emitted from the elements of the periodic table that
Moseley was studying. In this wavelength region prisms could not function, only
gratings could work and in particular the ones nature made in the form of
crystals, such as the crystal is shown below. This natural grating should
reside in a brightly lit gilded glass case on a pedestal just as
with Galileo Galilei's famous Lens sometime does. They both gave all of us a
better and very important view of the world we live in. Read on and see to see
Mothers nature spectroscope designed and built a few years before Newton
set us all straight about rainbows. Bless him.


Doesn't look like much does it? Not going to win any academy
awards will it? Galileo's Telescope and our replica of his telescope have stood
out his museum for the public to understand and learn from.
Everything else around it was supporting equipment.
There is some rumor that Moseley "borrowed" the crystal from the Bragg's
experiments as well as their
theory generated for the crystal. another hint about scientists they physically
and intellectually share their work.
On the
other hand THE PHOTO BELOW SHOWS Henry Gwyn Jeffreys Moseley as a
STUDENT WITH STUFF IN HIS HANDS GRABBED OFF THE NEAR BY LAB TABLE READY AND WILLING TO BE THE NEXT
MEDIA HERO TO BE CELEBRATED GOOD GUYS IN THE "Mystery of Matter". Moseley had another hero thing going for him. right after
his discoveries He was kill by
sniper fire in WW I. He than acted as sort of a poster scientist
recommending (by his absence )that scientists be relieved of active duty in time
of war. There were lots of talented people lost in that war. Another famous grad
student scientist Marie Curie saw the products of war, First Hand, by working
with her x-ray truck x-raying solder's
battle wounds during WW 1. Below is photograph of the Replica we
made of from one of Moseley apparatus.

The modern humans view of the world with
Young Moseley's help. With others he took on the x-ray region.
.jpg)
.jpg)
The object of Moseley's
method of research was to generate the spectra of each element to be measured
with all its electrons stripped from that element. All but one. From natures
point of view, that element then thought it was hydrogen therefore it would
give a hydrogen spectrum. It did ... but not exactly. The protons
pushed the spectra into the blue blue blue blue x-ray region of the
spectral world. The spectrum was pushed by the numbers of protons in the center
of the atom. This measurement technique could be done
experimentally from the spectrum generated by the magic crystal above. The
crystal that knew it was a spectra generating grating and that
it was not a prism at these short wavelengths. The
bottom line not only did it verify Bohr atomic theory of what an atom
looked like it gave a better reckoning of the atoms in the periodic table. All
this in doctoral theist. Madam Curie gained her Hero status in her doctoral
theist also also with a lot of support from colleagues.
Back to the picture of the humble nonchalant pose of Moseley grasping the
bits and pieces of stuff he had swept off the bench
for this hasty shot.
Seems that everyone uses this photo to sell their web site whether
the photo is relevant or not. Its a magnet. (sorry about
that.)
In particular note the circular pot out in front. It holds the All important measuring instrument , the x-ray diffraction crystal
spectrometer. The glass apparatus behind it holds the sample
and the its x-ray exciting electron gun. A simple 1/8 " piece of wire with a 3/4 " diameter
concave disk stuck on the ends of it to supple the electrons for the target .
One can note From the number of faces presented in the beginning Henry Gwyn Jeffreys Moseley
a grad student is not the sole Hero of these pages. He was a very
young man and had been encouraged by Lord Rutherford, Bohr,
consulted with the Bragg's (father and son), about a project.
Some implied data to this web site alerts those who want to get a more
basic understanding of how the team work approach in
experimental science really works.
Some of our work for this project was to get a clearer
first hand understanding of the Moseley experimental operation.
We show in full color the equipment that we (Jim and Rhoda
Morris) have built using NSF monies and donating much of our time replicating
Moseley experimental set up. We built a working instrument for a movie titled
Mystery of Matter. We have done this for one to see a little more into our
beloved science and how its team of individual competitive scientists work
really well together to get it right ... Quite incidentally ... for your
and our richer life. Most don't appreciate this and don't have the
inclination to care. It just happens.
We take on more step showing this team process. We
put forth ( with Moseley own words) describing this in his abstract,
of the famous paper where he is reporting his results.
Take the time to see how Moseley put his role in perspective to the
others scientist who he was working closely with.
Below the abstract of Moseley Paper
giving the credits due and his approach to the research.
|
THE HIGH FREQUENCY SPECTRA OF THE ELEMENTS
By H. G. J. Moseley, M. A.
Phil. Mag. (1913), p. 1024
In the absence of any available method of spectrum analysis, the
characteristic types of X radiation, which an atom emits if suitably
exited, have hitherto been described in terms of their absorption in
aluminium. The interference phenomena exhibited by X-rays when scatted
by a crystal have now, however, made possible the accurate
determination of the frequencies of the various types of radiation.
This was shown by W. H. and W. L. Bragg, who by this method analyzed
the line spectrum emitted by the platinum target of an X-ray tube. C.
G. Darwin and the author extended this analysis and also examined the
continuous spectrum, which in this case constitutes the greater part
of the radiation. Recently Prof. Bragg has also determined the
wave-lengths of the strongest lines in the spectra of nickel,
tungsten, and rhodium. The electrical methods which have hitherto been
employed are, however, only successful where a constant source of
radiation is available. The present paper contains a description of a
method of photographing these spectra, which makes the analysis of the
X-rays as simple as an other branch of spectroscopy. The author
intends first to make a general survey of the principal types of
high-frequency radiation, and then to examine the spectra of a few
elements in greater detail and with greater accuracy. The results
already obtained show that such data have an important bearing on the
question of the internal structure of the atom, and strongly support
the views of Rutherford and of Bohr.
Kaye has shown that an element excited by a stream of sufficiently
fast cathode rays emits its characteristic X radiation . He used as
targets a number of substances mounted on a truck inside an exhausted
tube. A magnetic device enabled each target to be brought in turn into
the line of fire. The apparatus was modified to suit the present work.
The cathode stream was concentrated on to a small area of the target,
and a platinum plate furnished with a fine vertical slit placed
immediately in front of the part bombarded. The tube was exhausted by
a Gaede mercury pump, charcoal in liquid air being also sometimes used
to remove water vapour. The X-rays, after passing through the slit
marked S in Fig. I, emerged through an aluminium window 0.02 mm.
thick. The rest of the radiation was shut off by a lead box which
surrounded the tube. The rays fell on the cleavage face, C, of a
crystal of potassium ferrocyanide which was mounted on the prism-table
of a spectrometer. The surface of the crystal was vertical and
contained the geometrical axis of the spectrometer.
In almost all cases the time of exposure was five minutes. Ilford
X-ray plates were used and were developed with rodinal. l
|
Going beyond just our young HERO ... Moseley ...
and his atom project, one has the chance to see here pictures and comments
showing that no scientist stands alone in any discovery. No one
scientist is a super being. Proper training, persistence, being at the right
place, right time, are all any scientist can hope for.
All scientists are surround by colleague's, who are both young and old painting
an emotionally stimulating, picture, of a spectrum of an internationally -
diverse group of hard working scientist.
Beyond many peoples belief that even though scientists - are fiercely
competitively, they work intensely together. Specifically they are on one
long term project which is hidden from direct view of the public but appears
( to some ) as taking a part of a - - never ending
paving - of a road - to make everyone's journey into the future
gentler and fuller.
This togetherness picture when ever painted
appears to be unbelievable by the general public. They view
scientist as just another group of themselves exhibiting all
the good and not so good ways of human nature. Few if any us have worked side by
side for or with a number senior performing scientist. Non of us will ever
have or want to have to do this for an extended length of time to
see what it is really all about.
Hmmmm just Hmmmm
Remember.
At the heart of young Moseley's instrument was a unique crystal grating
using Bragg's (fellow scientists) Law
to make an x-ray grating spectrometer. Prism's do not work in x-rays region of
the electromagnetic spectrum. Sorry Mr. Newton we can't use all that beautiful
work you published. You should have been on Young's view of electromagnetic
radiation.
Such an instrument was hovering around near
by in the Bragg clan laboratory area. They were interested in measuring
the arrangement of atoms in crystals.
Bohr who was around on and off visiting the neighborhood drawing
pictures of models of his atom ands making calculations of his arrangements of
the pieces in his model of the hydrogen atom using just the visible spectrum
data taken from lots of other scientists.
The latest periodic atomic
arrangement of atoms in nature suggested from Moseley approach and data
which he obtained from one or more of his grating spectroscopic
measurements.
.svg[1].png)
_______________________________________________________________________________
The following are some scraps and unfinished business in
this web site.
Below is a photo of a
capillary hydrogen light source viewed through a transmission grating. Each side
of the center pinkish red line which is the direct view of the hydrogen source
are the alpha beta atomic lines in the visible spectra.

The latest periodic atomic arrangement
of atoms in nature suggested from Moseley data which he obtained from one
or more of his grating spectroscopic measurements.
.svg[1].png)
The first Spectroscopic Analysis deriving the concept of Atomic Number.
Also Moseley's experimental verification or Bohr approach in describing
the structure of the atom.
This was a profound example in understanding how scientist work.
Which is never alone but a serial step by step of competitive corporation with
each other checking, cross checking each other, their models which they
pass on to the next group of scientist.
Making Replication of Moseley apparatus that he used for arranging - rearranging
the Periodic Table
Our contribution to this stew of science has been
acting as contributors to the Moseley story as part of NSF sponsored
documentary called The Mystery Of Matter. We were asked to replicate
his instrumentations. We had the pleasure of visiting Oxford University and take
measurement of what was left of his equipment. Having done this were in
the position to replica his equipment and experiments more faithfully.
By Jim & Rhoda Morris
http://www.scitechantiques.com
From the book of Genesis 1.3, God said "let there be light"-- And God saw that the light was good".
A scientist said "give me some electrons I'll push them
around and they will give us light. Is this good?
Moseley pushing electrons around and studying the light that they
gave off with his light measuring scientific instrument **The spectroscope**saw
that it is was good. Good for his reputation. It wasn't it good
for those scientist's reputation's who had put elements
in the wrong box of the periodic table.
They had not been using **
the spectroscope** to get the right answer. Was it good for
science's reputation showing that scientist could be
wrong? Was it good for the human race who gained a
better life with the "right; or almost right answer?"

Moseley used the same optical style of spectroscope
as pictured above, but he used a diffraction grating rather
than a prism. Both the prism and the grating divide up the light into
its colors but Prisms do not work in the x-ray region. Some X-Rays-
if they are strong enough - pass through a prism but without bending very much if at all
into their colors.
.
Moseley gathered his experimental data in x-ray region of
the spectrum using single crystals to divide up the light
into its colors. Earlier
than Mosley, Marie curie used x-rays to find bullets in soldiers
bodies in the war I. The same kind of bullets that ultimately would kill
Moseley who chose to fight in the war, which he used to correct
the mistakes others made in the placement of elements in the
Periodic table. He even predicted that scientist had been
missing elements in this table.
Planetary atom: Far more than the finding of empty holes
in a table of elements, Rutherford likened this kind of research as
stamp collecting. Moseley had used his spectroscopic data to
experimentally check, verify and select the interesting and then current
theoretical model offered to explain the nature of hydrogen
atomic spectra. The one he chose was the classic Bohr's picture of a planetary atom.
The theory in its simplest form fit hydrogen spectra which didn't last very long before it was
modified with a statistical approach. Still it was a
significant start.
Interesting planetary models were a hot topic in
Galileo's time to some and certainly to some historians looking for connections.
Galileo 300 plus years ago was struggling with another . The model
of our solar system. He was gathering acceptable data using a
critical component of a spectroscope-- the telescope in his work. See
the last picture in a collage of two replicas of
Galileo telescope made into a spectroscope with prism.
"Note" Simply replacing the prism with a rock salt
grating one could use this spectroscope in Moseley experiment today.
Science was on a roll around 1900's +- 25 years
when Moseley' appeared on the scientific scene.
In Moseley's time scientist had made enormous improvements in
scientific instruments that expanded human's sense ,of seeing, hearing, feeling, etc.,
millions of times greater than nature provided. Example humans in
Moseley time had to get acquainted with very tiny and very large
things at the same time not only single atoms but parts of the atom, electrons, that
they could not see directly. The
Lorentz radius of the electron, 2.8 * 10 ^-13 cm.. The diameter of
the nucleus of
hydrogen proton was in the range of (1.75×10−15 m)
On the larger size they have had to start dealing with a universe billions of light years in radius.
All this with the new scientific instruments with the little valued
spectroscope leading the way.
Gaining perspective of the instruments that Moseley's
had available to work with were improvements in vacuum
technology (from the lighting world), high powered high voltage power
supplies (from the power distribute system to light the world), new
and more precise spectroscopes (from the analytical chemical world).
Added to this arsenal was the statistical mathematics from the
(business world).
The hottest scientific topics were the discoveries
of particles that atoms were made of electrons protons x-rays
and especially Marie curie's discovery of the enormous energy of
the nucleus and Einstein's E=mc^2 were keeping the world of science
flat out.
Moseley luck and borrowed gifts from many others was his
fascinated with high vacuum pumps , high voltage electron beams
and the x-ray grating spectroscopes.
For the latter if we go back in time one of the simplest examples
of humans relationship with spectroscopes, were nature's rainbows.
Humans could only view the sun's radiation using their natural senses.
They noted that
exposure to sun meant sunburns - later refer
to as ultraviolet radiation, the visible perception colors
light like the
rainbow, the sun's warmth later called infrared
radiation.
In our ,new, world
Moseley decided with help from his adviser to use a famous
senior scientist, Dr. Bohr, last gasping attempt to use classic
mathematical methods to build a formula to predict
the spectral light coming from very hot electron
temperature) hydrogen plasma. Moseley using this incorrect
(in the quantum mechanical sense) theory decided to count the number of individual protons in atoms
using man made rainbow generators (grating spectroscope made
using a large crystals grown from table salt as the light
dispersing element ). His goal was to correct the chemist's catalog of
elements and the pieces they are made of, appearing
(15,000 K)in the Period Table.
Again Quoting from Genesis
In the beginning "and darkness was upon of the deep'" and "and God
said" let there be light"- In science the rainbow spectroscope was
showing scientist the way to go both big and little with the
planetary model. |
The cave mans view of his world.

|
Nature's spectroscope; using spherical ( oblate spheroid
to be more exact) rain drops Light from the sun passes thru rain
drops forming a spectrum which we call a rainbow. Note in the photo
hidden from the human eye are radiations beyond the red and blue. -Unfortunately
to some?- humans could only see a tiny portion of nature's infinite number of colors.
Figure 1 below shows how the visible spectrum connects with the rest
of what scientists now call the electromagnetic spectrum. between the
names radio
frequencies, visible, and gamma rays.
The point here is to note that there is this limitation in all
our senses ;
light, hearing, touch, smell, etc. Some would even include common
sense in the list? -Fortunately?- scientist have extended all of
our physical senses , perhaps with exception of one, with scientific instruments to expand our
exploration of the world.
The Electromagnetic spectrum--
The figure below may seem complex to many people. The names do not seem
connected. This is because as the scientist we're exploring each new radiation
they needed a unique detector they gave that portion of the electromagnet
spectrum a unique name. In the final cut the only difference is in
the size of the wavelength or the frequency of the radiation.
|
The modern humans view of the world
.jpg)
figure 1
|
Nature has determined that all elements when heated to a high
enough temperature will give off and or absorb radiation (each
with a unique rainbow) they appear in
very special places spread about the electromagnetic spectrum.
Scientist call these "rainbows" "spectra. It helpful to many to
think of spectra as barcodes because spectra look and act just like
barcodes. A special example for our purposes in replicating Moseley work on the
periodic table we show the reader the spectra Moseley considered to be most
important was the simplest element of them all hydrogen. Again, Moseley decided to make all
element act with hydrogen because hydrogen's spectra is simple
and most important forecastable . One of the spectra that hydrogen gives off "light"
is in the visible. It almost a poetic in shape as shown below).
It has
a pair of lines red an blue followed by clump lines quickly gathering together in
the blue and deep blue to form almost a continuum. For our convenience
we do not show the thousand and thousands lines bunched up here
in the deep blue. This spectra had been labeled by a number of
scientist around Balmer time as the Balmer Series. It shows up
in the visible. Another set is similar is the configuration the Paschen
series appears in the infrared and a third Lyman Series in the
super Blue. |
Top spectrum in the figure below shows the strongest
visible lines of atomic hydrogen.
The spectrum below is hydrogen like but in the x-ray region which is
invisible to the human eye. Theselines are easily photograph however .
Note the strong similarity of the on top and bottom spectra. Actually their is
one small change in the lines on the photographic plate. They have been
enlarged wavelength to show their similarity. The reason the hydrogen like
lines are in the x-ray region they are not from hydrogen. They are
from heavier elements that have had all of their electron shot away with
stronger electrons. This leaves the nucleus in tack but strongly positive. The
first electron to come back to the element gives a hydrogen like spectra
but in an x-ray region. By measuring the wavelength one has measured the
number of protons in the nucleus which is the atomic number.is waiting to
be places in the period table.
.jpg)
|
Summary
All elements can be electrically excited to produce a hydrogen like spectra
by simply stripping off all of its electrons. Moseley did this by
shooting electrons generated from a high voltage source at a target
of the atoms being studied. This generated a source of the ionized
elements that would produce a one electron type hydrogen spectra however
they will be shifted toward shorter
wavelengths in the x-ray spectra due to the higher number of protons in the element. One
can use this spectral shift to measure the element's atomic number, Z (its
number of protons). Shown above is the spectrum for hydrogen and the
corresponding hydrogen like spectra for the test element being measured in the
x-ray region.
Moseley and fellow scientists devised
equipment and experimental methods in spectroscopy to include the x-ray
wavelength region of the spectrum, the region necessary for measuring
the number of positive charges in the nucleus of atoms. By accurately measuring
this unique number (known as the atomic number, Z) for each element they were
able to accurately determine the location of an element in the periodic table
and even predict empty slots and what the element might act like . This
table is one of the most important catalogs or spreadsheets for science
and engineering.
Moseley's work was far more reaching than the
periodic table. His work helped give experimental verification of models
explaining what an atom might physically look like, namely Bohr's planetary
model of the atom. Below one of a number Moseley's later instrument used to
measure the atomic number of the elements.
Below is one of a number of
experimental set ups used to in Moseley's
studies.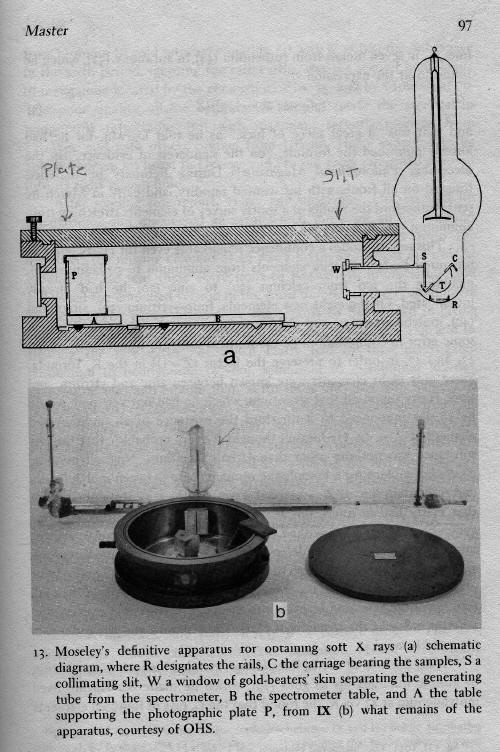
Below is a our replica of the glass and
metal components shown in the picture above.
|
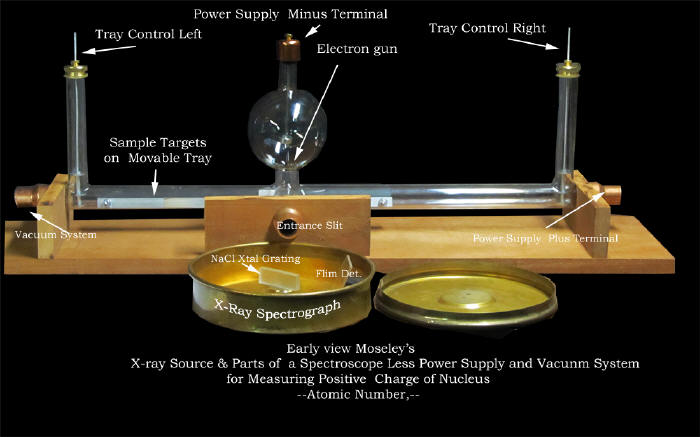
|
By producing hydrogen like
spectra
from totally ionized atoms of a relatively large number of
elements in the x-ray region Moseley found a method to accurately
position the elements in our periodic table. |
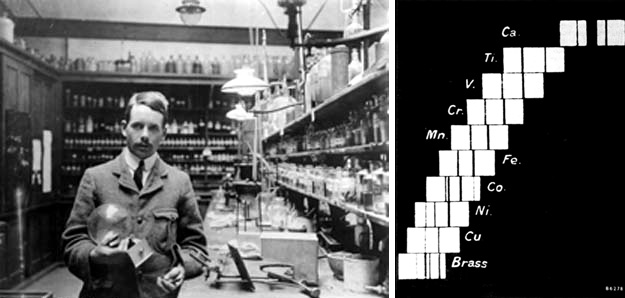
|
To the left is Moseley and some of his apparatus. To the
right in the figure above is Moseley's hydrogen like spectra showing the
alpha and beta spectral lines for variety of elements including Copper,
Nickel, Cobalt, Iron, Manganese, Chromium, Vanadium, Titanium, missing element,
Calcium.
Below: We generated the atomic spectrum of hydrogen from a low pressure gas
discharge tube for readers satisfaction at the simple yet complex spectral
features Moseley had to deal with in his experiment. We show what happens when
one looks through a grating spectroscope at hydrogen heated by electrons in what
scientist call in this instance a gas discharge tube, a very important accessory
in science. Moseley used a source of electron also but bombarded solid
targets to strip all the bound electron of the atoms. As the electrons
rejoined the atom the first ones generated a line spectra similar to a
hydrogen spectra but in x-ray region located by the number of protons in the
atom.
In the figures below---Looking through a transmission
diffraction grating at the discharge tube, one will see in the center, the primary non refracted image of the discharge tube. On each
side of this center image are the images of the diffracted first and second
orders of the hydrogen alpha and beta spectral lines. There are more spectral
lines (see figure above) too faint to be recorded by the camera. This was the
true of Moseley's x-ray spectrograms. This spectrum shows the skill
Moseley needed to separate the parts of the spectrum need for the
measurement.
|
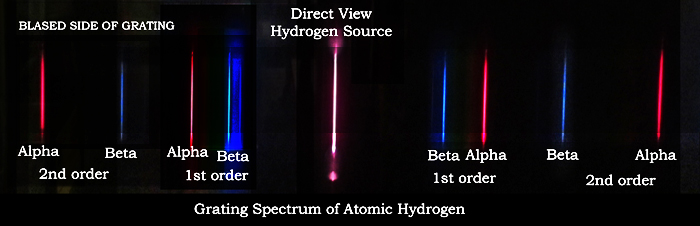
|
Spectra of atomic hydrogen as viewed
through a diffraction grating |

|
The hydrogen low pressure arc source. |

|
The diffraction grating in the
foreground the arc source in the background |

|
The light source as seen looking through the grating
----------------------------------------------------------------
The collage in the photo shown below shows one
of the greatest irony of science. Galileo had in his hands all
of the basic components to build a spectroscope of one of the most
important scientific instruments invented. It took nearly 300 years
and a frog to bring the rest of the equipment
needed up to speed to see "a atomic
planetary " system.. Planetary, a concept, a word, challenged by a Catholic church only
to be replicated in atomic dimensions. Protons
circled by electrons. In this case the planets
(electrons ) are giving off the light. not the protons (the sun). |

-----Rhoda and Jim comments----Oh how slow some instruments take to be
developed. Two very precise replicas of Galileo Telescopes turned into one of
the most powerful scientific instruments of all times. It took nearly 359
years to add a simple prism and input slit to discover the tremendous
value such a simple instrument. The most important missing link was the battery
which allowed us to heat up atoms and molecules hot enough to generate their
spectra. We also needed a good vacuum system to achieve low pressures for
exciting even higher temperature to see ionic spectra. The spectroscope has given us quantum mechanics,
shown us the size and content of the universe etc..
Below the abstract of Moseley Paper
giving the credits due and his approach to the research.
|
THE HIGH FREQUENCY SPECTRA OF THE ELEMENTS
By H. G. J. Moseley, M. A.
Phil. Mag. (1913), p. 1024
In the absence of any available method of spectrum analysis, the
characteristic types of X radiation, which an atom emits if suitably
exited, have hitherto been described in terms of their absorption in
aluminium. The interference phenomena exhibited by X-rays when scatted
by a crystal have now, however, made possible the accurate
determination of the frequencies of the various types of radiation.
This was shown by W. H. and W. L. Bragg, who by this method analyzed
the line spectrum emitted by the platinum target of an X-ray tube. C.
G. Darwin and the author extended this analysis and also examined the
continuous spectrum, which in this case constitutes the greater part
of the radiation. Recently Prof. Bragg has also determined the
wave-lengths of the strongest lines in the spectra of nickel,
tungsten, and rhodium. The electrical methods which have hitherto been
employed are, however, only successful where a constant source of
radiation is available. The present paper contains a description of a
method of photographing these spectra, which makes the analysis of the
X-rays as simple as an other branch of spectroscopy. The author
intends first to make a general survey of the principal types of
high-frequency radiation, and then to examine the spectra of a few
elements in greater detail and with greater accuracy. The results
already obtained show that such data have an important bearing on the
question of the internal structure of the atom, and strongly support
the views of Rutherford and of Bohr.
Kaye has shown that an element excited by a stream of sufficiently
fast cathode rays emits its characteristic X radiation . He used as
targets a number of substances mounted on a truck inside an exhausted
tube. A magnetic device enabled each target to be brought in turn into
the line of fire. The apparatus was modified to suit the present work.
The cathode stream was concentrated on to a small area of the target,
and a platinum plate furnished with a fine vertical slit placed
immediately in front of the part bombarded. The tube was exhausted by
a Gaede mercury pump, charcoal in liquid air being also sometimes used
to remove water vapour. The X-rays, after passing through the slit
marked S in Fig. I, emerged through an aluminium window 0.02 mm.
thick. The rest of the radiation was shut off by a lead box which
surrounded the tube. The rays fell on the cleavage face, C, of a
crystal of potassium ferrocyanide which was mounted on the prism-table
of a spectrometer. The surface of the crystal was vertical and
contained the geometrical axis of the spectrometer.
In almost all cases the time of exposure was five minutes. Ilford
X-ray plates were used and were developed with rodinal. l
|
|








.jpg)
.jpg)
.svg[1].png)









