|
Priestley's Burning Lens and
Experiments Generating oxygen
Some early replications of Priestley's chemical work including
comments on solar heating and decomposition of mercuric oxide.
Jim & Rhoda
Morris k1ugm@comcast.net 781 245 2897
SciTechAntiques.com
|
In the beginning God said let there be light.
Priestley took God up on this gift used sun light (the violet
visible and near infra red) captured by a lens that focused an image
of the sun on a sample of
mercury oxide which was contained in a glass vessel. This was a form of
pristine clean heat that could bring a sample to temperatures
greater than 500 C. decomposing HgO it into pure oxygen and mercury
vapor. The mercury vapor was condensed to room temperature pressure
by passing through a water bath. Prtiestley then used the oxygen for a number of
experiments which included the very important study of the
affect of oxygen and carbon dioxide on plants and animal life He
found that each fed on the waste products of the other. A discovery of
immense importance to our future energy balance
To rerun the animation hit refresh button. F5 on explorer. |
 |
|

This photo shows a likely replica stand that Priestley would have
used. It has a temporary lens rim holder, etc. It had
not received its final finish at the time of the photo This
lens is 10 inches in diameter and has a 24 inches focal length.

Some a quick tests were made with the sample held in a 2
cm dia test tube. Note the image of the sun has overloaded the
camera and the sample appears white. The sample is really dark brown
to black. to the eye. The only change is when it
reaches 500C - it simply disappears. The correct
exposure for the sun's image is not the optimum exposure for the
sample next to it. Its intensity can be compensated to get both but
it takes care. This set up was used to test the color change
by heating the sample container with a torch instead of the sun.
it did as is described in the right hand column starts off. red
turns black simply disappears. However;
The whole affect of the red powder scattered
about the inside of the glass, the silver-ish mercury
droplets clinging to the glass surface giving it cloud like sheen,
the bright image of the sun if it is moved about when held by the
hand as Priestley probably did, can and does make a dramatic
surrealist hellish Mars type scene see below. and all of this is
real with practice.
A note of caution about
mercury:
Mercury vapor is not good for your health.. Depending upon
room temperature and poor ventilation there can be low but
dangerous amounts of mercury inhaled which if integrated
over long exposure times is not good A very rough guide:
exposure time * amount = trouble
Most of the mercury vapor is condensed in the sample vessel and
the residual is trapped in the water bath. So as long as
the gas tube to the trough is intact when the vessel is at high
temperatures, there should be no exposure problem.
Notes of caution about the sun's image from the burning lens:
Suitable eye protection is needed
One must be careful staring at the image of the sun on the sample.
It is very bright. When you look away your center vision will be
somewhat impaired.. This will be for a given length of time,
depending on your age. It can be from seconds to minutes. It might
take several minutes for you to be able to regain your normal
vision.
Caution - It can take a minute or more to adjust the sun's
image on the sample and if care is not taken one might accidentally
get the image of the sun cast into the eyes.. Proper glasses have to
be provided to protect the operator from the sun's high intensity
infrared radiation which can burn the retina without the person
feeling any pain. This is the same warning for protection
given to viewers for solar eclipses.
The plano-convex lens used in these Priestley experiments has
a nasty feature in that it forms a reflected image of the sun in
front of the lens. That is on the suns side of the lens. It's only four percent-ish of the real
image but should be treated with care to protect people's eyes. It's
sitting out there and you can not see it!
The other danger can result from improper storage of the lens.
It can cause a fire inside or outside if stored such that it images
the sun on a flammable substrate. |
|
Jim & Rhoda's sketchy notes/
principally for themselves
Warning!: This document may not be comfortable
for those who cherish rules of grammar, spelling, punctuation,
organization and style more than content. but others can browse with
leisure.
Priestley project on HgO - Not for publication.
He generated oxygen a number of different
ways. The principal process was by heating a sample
using a lens to concentrate an image of the sun on mercuric oxide
(or what ever)
.Priestley had to learn more than a bit about
optics that was not published in a text book to
effectively learn how to optimize his lens location etc for the most
effective heating by the lens method. One does not just stick up a
lens and go at it to replicate his experiments. The following are
some of the pit falls one can fall into.
What happens to the sample of mercuric oxide
as it is heated and what do observers see? What can a
camera record?
As the sample is heated it changes from an orange red at room
temperature to near black in the neighborhood of 400 C. Then,
within a few degrees of 500 degrees C it simply disappears by
decomposing. The decomposition products are mercury vapor and
oxygen-both colorless gases. Most of the mercury vapor soon
condenses into very tiny droplets on the cooler parts of the
inner walls of the container. The vapor can be harmful if inhaled
when it is not removed by cooling it properly. The mercury
condensed on the walls of the glass vessel, of course, obscures the
sample from the sun's rays and the camera viewing the sample.
Note:
Remember to pre-heat the container wall through which the camera
views the sample.. Purpose; to reduce the amount of mercury
condensing on that part of the container wall.
It will be useful to note that the ratio of the
brightness of the sun's image on the sample is several
orders of magnitude greater than the surroundings. This makes it
taxing for most cameras or displays to accurately portray how the
sample is decomposing in the sun's image. The suns image is just
white.
In general its a challenge to use a burning lens
quantitatively.
Basics of solar radiation.
1, About half of the energy radiated by the sun is beyond the
wavelengths we see the in the visible image of the sun. Therefore
the visible image is not the best indicator of the total heat
available. There will be days when the sun and its image will appear
to be bright but a good share of the infrared radiation can be
missing through scattering and transmission losses of the
intervening air.
The absorptance of the surface of a sample is a critical
factor of how much radiation and will be absorbed. It depends in the
1st order of the absorptance of the sample. ??
2, The condition of any optical surfaces
between the lens and sample may adversely affect the amount radiant
heat getting to the sample.
3, If the lens is not
perpendicular to the optical axis this will cause image distortion
and an unfavorable distribution of the thermal radiation getting to
the sample. On the other hand it makes for some weird
interesting images during focusing.
Surprise Last but not least because there can be a
significant problem with chromatic aberration with the lens the
location of infrared image of the sun will not be where the
location of the visible image is. The different between these
two images can be as much as 1 to 2 inches from
the lens. The result you may have a sharp image of the sun in the
visible and not be focused for the infra red image where much of the
heat is. located..
The photos below were taken while subjecting the HgO
samples to either one or both sources of heat; an
external one such as a small electric or gas torch, the other an
internal source from radiation absorbed from the sun through a
burning lenses.
We also include here photos of samples exposed to
the burning lens and various views of the operator in
and around the experimental apparatus to expand the opportunities
for visual affects.
|

( click on the photo to enlarge the interesting detail)
Testing out decomposition of the HgO with the camera above
and shooting thru another large lens. Note Rhoda is cooling
most the residual mercury vapor in the a water bath keep most
of it out of harms way. No, this is not the flask intended for the
shoot.

(Click on the photo to enlarge the interesting detail that can be observed
if the camera can move in on the scene close enough ) Above is the sample
in a 500 cc test flask ,similar to the retort to be used during the
filming, being heated by sun light through the burning lens . As the
sample of HgO heats up, it turns black-ish, and disappears as the
mercury and oxygen separate. The inside of the wall of
the flask, which is cooler, as we noted before, clouds up with
condensed mercury vapor building up more and more with time until it is
nearly opaque. It can be removed by external heating. which could be done
with a gas or electric torch. This so called window area can be
kept clear of condensate by pre-heating this area before the application
of the sun. The bright spot on the right is the sun's reflection off
the glass and on the left off the mercuric oxide powder. The sample
spot appears white because of over exposure but it is really
blackish if properly exposed. In some ways these tiny droplets
of Hg on the surface of the vessel seem to add to the drama in
the scene.
Getting a repeatable visual affect of this mercuric oxide decomposition
process is very touchy but do-able. The visual affect can depend on the
history of the local environment, i.e. previous conditions such as, where
and how the application of heat was previously applied, where
the sample was located in the vessel etc. Producing and collecting oxygen
is easy compared to documenting the process
Collecting the oxygen
Priestley directed the oxygen he generated into a water
displacement trough like shown below. The oxygen was fed through
a flexible tube into an invert jar filled with water that had been
place on a shelf in the trough.. The gas bubbled out of the other
end of the tube into an inverted jar displacing the water inside it
with oxygen . See the lower right hand picture below.
Priestley had any number of these troughs made for him.. Our replica
is the size of one of them. |
Below a collection of photos of opportunity
 |
 |
| This is not a staged scene . Rhoda is surrounded by
the equipment clutter and most times it's worse. It was probably
the same for Priestley's lab table except for the age of some of the
articles. Experiments are usually messy - a mixtures of many trial and
errors of more than one test.. |
Yes these are not period glasses. Priestley probably
used a smoked piece of glass to view the bright sun images.
These could easily be made by holding a piece of glass over a candle
flame. |

Looking through the lens at the experiment some distance from the
lens..The oxygen generated is collected by water displacement in a
trough but the gas includes some non-condensed mercury vapor
which is filtered by the water, an added safety feature.
The tube leading from the retort to the oxygen collection glass should
not be removed from the water until the flask is cooled to room
temperature and a well ventilated room should be used for the
experiment..

Just another view showing the diversity of uses of the lens. |

As the sample is being heated by the image of the sun it can be seen
reflected off the surface of the lens superimposed on the operator who
is adjusting the image of the sun onto the sample. It is an
interesting view and Rhoda is in a safe position.
But some caution should be taken for the one taking the photo.
Again repeat of Caution (as given above)- it can take a minute
or more to adjust the lens to cast the sun's image on the
sample and if care is not taken one might get in the wrong place and
accidentally get the image of the sun cast into the eyes.
To repeat: The plano-convex lens has a nasty feature that if
forms a reflected image of the sun in front of the lens. It's only
four percent-ish of the real image but should be treated
with care. Storing the lens in a safe location so that it is not
imaging the sun or near flammables, is also important.
Proper glasses should be provided to protect the operator
from the sun's high intensity infrared radiation which can burn the
retina without the person feeling any pain. This is the same
warning for protection given to viewers for solar eclipses. |

Another photo showing the reflected image of the sky etc from the
surrounds
Lens 10 inches in diameter 24 inch focal length. Samples were held in
500 cc. flask. |

One of our original setups used an 8 inch diameter lens cut to a
square format. It has a 12 inch focal length. Samples were held in a
test tube rather than a flask. We were able to use a 2
inch diameter lens with a 4 inch focal length to decompose the mercury
oxide in even earlier experiments. The lens size made little
difference; it just effected the size of the sample that could be
.decomposed. Just a simple demonstration that the "F number" is
the all important specification of the lens used in Priestley's
work, not the size. Confusing? Yep. Important? Yep!
only to the techs though. |
The pictures below show how the shape of the
sun's image is altered as a lens is rotated away from the optical axis.
This could be an interesting feature that can be used while the operator
is setting up the lens for the experiment. It also shows that the heating
effect of the sample can be reduced by improper adjustment of the lens.

Image of sun lens properly adjusted perpendicular to optical axis. |
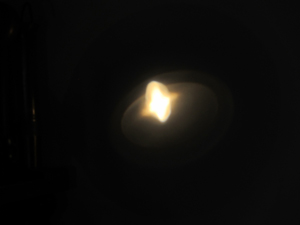
Lens rotated 10° from axis. |
|

Lens rotated 60° from axis.
|

lens rotated 80° from the axis. |
A note of detail & interest--- I would wonder about even the
existence of the so called big lens that Lavoisier was supposed to have
used? There should be reasonable doubt that anyone could have poured
such a large piece of glass without cracking it during the cool down
process which would have taken months. (Remember the trouble
Priestly had heating and cooling little pieces of glass. Hale had
big trouble, with the 200 inch telescope, even when Pyrex was used
it took more than a year to cool it without cracking it - let
alone grinding and polish it to any useable figure!!)
Side Note
Too bad that their is not enough time to open the scene with a full screen
image of one of NASA motion mixtures of the sun in Ca or Na light. Better
to include the movies with the turbulence images. There are
those practicing scientist including a Nobel prize winners that
suggest strongly that at least 5 scientist contributed to the discovery of
oxygen over at least 5 years. Rhoda and jim have no stand on this subject
nor a Noble prize.
Random Note
This all may seem disconnected but it not it is how science works bit by
bit by bit. most time it takes along time to get these connections.
The following web page dealing with the long scale is not
directly a Priestley topic and can be skipped. It for those of who
are apposed to global climate change. and have just enough knowledge of
the subject to have badly miss read the counter physics of it
Admittedly, when the reader gets to it, the
scale below is long, but it's to demonstrate that it is actually quite
short, compared to full output of the sun. It also contains the
transmission of the earth's atmosphere. At the dips the atmosphere
absorb the sun's energy and heats up just as the mercury oxide does in its
container. If we were to draw the whole thing (for all
wavelengths) it would extend from near zero a very large
number, without calculating it perhaps several feet.
This is the sort of data
of interest to global climate change issues. The wave length is in
microns. Note how tiny the visible spectrum take up out of the more or
less total spectrum. It has been a long time between Priestley and
his companions used the power of the sun for their work and the generation
of the scale below but he and his gang help start us down the path. They
are like prospector looking for gold some only finding pieces here and
there but missing the seam.

|








